This work may not be copied, distributed, displayed, published, reproduced, transmitted, modified, posted, sold, licensed, or used for commercial purposes. By downloading this file, you are agreeing to the publisher’s Terms & Conditions.
Original Research
Lemborexant and Daridorexant for the Treatment of Insomnia: An Indirect Comparison Using Number Needed to Treat, Number Needed to Harm, and Likelihood to Be Helped or Harmed
Published: October 2, 2023
ABSTRACT
Objective: To determine if there are differences in the number needed to treat (NNT), number needed to harm (NNH), and likelihood to be helped or harmed (LHH) between lemborexant and daridorexant and to compare lemborexant with daridorexant indirectly.
Methods: Dichotomous efficacy and tolerability outcomes reported for Phase 3 daridorexant trials (conducted May 29, 2018–May 14, 2020) for months 1 and 3 were identified from published literature and regulatory documents. Analogous data were extracted for lemborexant from Phase 3 studies (conducted May 31, 2016–January 8, 2019). NNT, NNH, and LHH were then calculated.
Results: Lemborexant 5 mg and 10 mg had clinically relevant therapeutic effect sizes, evidenced by most NNT values versus placebo < 10 for Insomnia Severity Index [ISI], subjective total sleep time [sTST], and polysomnography outcomes. NNH values for adverse events (AEs) were > 10, suggesting relative tolerability. Somnolence was the most common AE. Discontinuation rates of lemborexant because of an AE were low, including for somnolence. Efficacy outcomes for daridorexant 25-mg and 50-mg doses pooled resulted in most NNT values versus placebo ≥ 10, with more robust NNT estimates for the 50-mg dose than for the 25-mg dose. Discontinuation rate because of an AE at month 3 was higher for placebo than for daridorexant, rendering favorable LHH calculations. Daridorexant evidenced low rates of somnolence or fatigue.
Conclusions: In Phase 3 trials, the benefit-risk ratios for both lemborexant and daridorexant were favorable as measured by NNT, NNH, and LHH. Indirect comparisons of lemborexant with daridorexant suggest an efficacy advantage for lemborexant and a tolerability advantage for daridorexant.
Clinical Trials Registration: NCT02783729, NCT02952820, NCT03545191, NCT03575104
J Clin Psychiatry 2023;84(6):23m14851
Author affiliations are listed at the end of this article.
Insomnia disorder is characterized by ongoing difficulties with initiating or maintaining sleep occurring at least 3 times per week, persisting for at least 3 months, and associated with daytime impairment.1 Insomnia is commonly reported in the general population and is often chronic, persisting for years.2,3 Additionally, insomnia is a regularly reported comorbidity with other medical and psychiatric disorders.2 It is also a risk factor for a wide range of disorders, including depression, anxiety, suicidality, Alzheimer’s disease, chronic pain, and cardiometabolic disease.3–5 Whether insomnia is reported independently or in conjunction with another condition, treatments aim to improve sleep quality and quantity as well as improve daytime impairments.6 Treatment strategies may include nonpharmacologic options and pharmacologic approaches.1,3 Recommended first-line treatment is a nonpharmacologic option—cognitive-behavioral therapy for insomnia (CBT-I). However, access to CBT-I may be limited, and there is heterogeneity in CBT-I treatment response depending on disease phenotype.7,8 A variety of pharmacologic treatments are available, which target a range of receptors including γ-aminobutyric acid A (GABAA), melatonin, histamine, and orexin receptors.9 The medications that target orexin receptors constitute a new generation of agents that specifically target the excessive wakefulness and arousal signaling observed in people with insomnia.1 To date, 3 different dual orexin receptor antagonists (DORAs) for the treatment of adult patients with insomnia characterized by difficulties with sleep onset and/or maintenance have been approved by the US Food and Drug Administration (FDA): suvorexant in 2014,10 lemborexant in 2019,11 and daridorexant in 2022.12
In the absence of direct head-to-head clinical trial data, it can be challenging to understand the potential therapeutic role for the different treatment options available, especially for new agents that may be unfamiliar to clinicians and patients. A possible solution to better inform clinical decision-making is the calculation of effect sizes such as number needed to treat (NNT) to describe benefit (therapeutic response) and number needed to harm (NNH) to describe untoward events such as an adverse event (AE) or discontinuation due to an AE.13–15 The ratio of NNH to NNT can further describe the benefit-risk ratio and is called “likelihood to be helped or harmed” (LHH).14 A recent report described insomnia treatment options using NNT, NNH, and LHH and compared lemborexant to other hypnotic agents, including suvorexant, doxepin, ramelteon, zolpidem, eszopiclone, zaleplon, and selected benzodiazepines.16 With daridorexant now available, the aim of this study is to describe the relative efficacy and safety of lemborexant and daridorexant using the metrics of NNT, NNH, and LHH.
METHODS
Data Sources
Categorical data from pivotal trials of daridorexant (NCT03545191, NCT03575104) were identified and extracted from product labeling,12 FDA regulatory Drug Approval Packages,17 and published literature.18 Corresponding data were then extracted from published and unpublished data of Phase 3 randomized placebo-controlled trials of lemborexant—the SUNRISE 1 (NCT02783729)19 and SUNRISE 2 (NCT02952820)20 trials. As documented in the source publications, all studies included in this analysis had protocols approved by the relevant institutional review boards or ethics committees and were conducted in accordance with the Guideline for Good Clinical Practice and the Declaration of Helsinki, and all participants provided written informed consent.18–20
Description of Studies
Lemborexant Study: SUNRISE 1 (Study E2006-G000-304, NCT02783729, EudraCT 2015–004347-39). The SUNRISE 1 Phase 3 clinical study was a 1-month, global, randomized, double-blind, placebo- and active comparator–controlled trial conducted from May 31, 2016, to January 30, 2018, at 67 sites in North America and Europe. A detailed description of the study design has been published previously.19 Briefly, eligible participants (women aged ≥ 55 years, men aged ≥ 65 years) met Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5),21 insomnia disorder criteria characterized by sleep maintenance difficulties (with or without sleep onset difficulties) that were confirmed using sleep diary, sleep history, and polysomnography (PSG). Insomnia Severity Index (ISI)22 scores were required to be ≥ 13. Following a 2-week placebo-only run-in treatment period, participants were randomized 5:5:5:4 to 30 days treatment at bedtime with lemborexant 5 mg, lemborexant 10 mg, zolpidem extended release (ER) 6.25 mg, or placebo. The primary endpoint was the change from baseline in latency to persistent sleep (LPS) for lemborexant therapy versus placebo, measured using PSG after nights 29 and 30 of treatment. PSG measures were recorded separately and averaged across pairs of consecutive nights.
There were 1,006 participants randomized (lemborexant 5 mg, 266; lemborexant 10 mg, 269; zolpidem ER, 263; placebo, 208) with a median age of 63 years (range, 55–88 years); 869 participants (86.4%) were women. Both doses of lemborexant therapy improved the primary endpoint, objective sleep onset compared with placebo as assessed by LPS.
Lemborexant Study: SUNRISE 2 (Study E2006-G000-303, NCT02952820, EudraCT 2015–001463-39). The SUNRISE 2 Phase 3 clinical study was a 12-month (placebo-controlled for 6 months), global, randomized, double-blind, parallel-group trial conducted from November 15, 2016, to January 8, 2019, at 119 sites in North America, Europe, Asia, and Oceania. A detailed description of the study design has been published previously.20 Briefly, eligible participants (aged ≥ 18 years) met DSM-5 insomnia disorder criteria characterized by sleep maintenance difficulties and/or sleep onset difficulties, which were confirmed using sleep diary, sleep history, and questionnaires. ISI scores were required to be ≥ 15. Participants were randomized 1:1:1 to treatment with lemborexant 5 mg, lemborexant 10 mg, or placebo at bedtime for a 6-month placebo-controlled treatment period. The primary endpoint was mean change from baseline in subjective sleep onset latency (sSOL) at the end of month 6.
For the placebo-controlled period (ie, first 6 months), there were 949 participants randomized in the full analysis set (lemborexant 5 mg, 316; lemborexant 10 mg, 315; placebo, 318), with a median age of 55.0 years (range, 18–88 years); 647 participants (68.2%) were women. Both doses of lemborexant therapy significantly improved the primary outcome, sSOL, compared with placebo.
Daridorexant Study 1: NCT03545191 (Study ID-078A301). This Phase 3 clinical study was a global, randomized, double-blind, placebo-controlled, parallel-group trial conducted from June 4, 2018, to February 25, 2020, at 75 sites in North America, Europe, and Australia. A detailed description of the study design has been published previously.18 Briefly, eligible participants (aged ≥ 18 years) with DSM-5–diagnosed21 insomnia disorder that was moderate or severe in intensity (ISI score ≥ 15) were randomized 1:1:1 to daridorexant 25 mg, daridorexant 50 mg, or placebo every evening for 3 months. The primary endpoints were PSG-measured change from baseline in wake after sleep onset (WASO) and LPS at month 1 and month 3.
There were 930 participants randomized (daridorexant 25 mg, 310; daridorexant 50 mg, 310; placebo, 310); 364 participants (39%) were aged ≥ 65 years, and 624 participants (67%) were women. Both doses of daridorexant significantly improved the 2 primary endpoints, WASO and LPS, at month 1 and month 3 compared with placebo.
Daridorexant Study 2: NCT03575104 (Study ID-078A302). This Phase 3 clinical study was a global, randomized, double-blind, placebo-controlled, parallel-group trial conducted from May 29, 2018, to May 14, 2020, at 81 sites in North America, Europe, and Asia. A detailed description of the study design has been published previously.18 Briefly, eligible participants (aged ≥ 18 years) with DSM-5 diagnosed insomnia disorder that was moderate or severe in intensity (ISI score ≥ 15) were randomized 1:1:1 to daridorexant 10 mg, daridorexant 25 mg, or placebo every evening for 3 months. Primary efficacy endpoints were as described for Study 1 (NCT03545191). The 10-mg dose investigated in this study is considered subtherapeutic and is not an FDA-approved dose, and thus it was not included in this analysis.
There were 924 participants randomized (daridorexant 10 mg, 307; daridorexant 25 mg, 309; placebo, 308); 363 participants (39%) were aged ≥ 65 years, and 638 participants (69%) were women. Treatment with daridorexant 25 mg significantly improved WASO at month 1 and month 3 compared with placebo. Daridorexant 25 mg did not improve LPS at month 1 or month 3 compared with placebo.
Outcomes
The selection of the categorical efficacy outcomes of clinical interest that were examined for this analysis was based on publicly available data for daridorexant17,18 and when the same measures were used in SUNRISE 1 and/or SUNRISE 2 (Supplementary Table 1).
Reported data for daridorexant included subjective response defined by total ISI score (ISI score < 10 [subthreshold insomnia], ISI score ≤ 7 [absent insomnia], and ISI score ≥ 6-point decrease from baseline [clinically relevant improvement]). Response was also reported for subjective total sleep time (sTST), as defined by > 80-minute increase from baseline. Data for these outcomes were then extracted from the lemborexant clinical trial database for SUNRISE 1 and SUNRISE 2, by study arm, at the same time points as reported for the registrational studies of daridorexant (month 1 for SUNRISE 1, months 1 and 3 for SUNRISE 2). The population (denominator for calculation) was the number of randomized participants who received ≥ 1 dose of study drug and had ≥ 1 post-baseline assessment on the efficacy outcome of interest. Categorical outcomes for ISI score ≤ 7 and ISI score ≥ 6-point decrease from baseline were previously calculated and reported for lemborexant.16
Regarding objective outcomes, PSG measures of LPS and WASO for daridorexant studies were reported in regulatory documents at month 1 and month 3.18 Categorical response thresholds included ≥ 50% improvement from baseline and ≥ 75% improvement from baseline for both LPS and WASO. Corresponding objective PSG response at month 1 was assessed for the SUNRISE 1 study from baseline (averaged from the testing done during the run-in period before receiving randomized study medication) to the end of treatment (day 29 and day 30 averaged). PSG was not conducted in SUNRISE 2.
Tolerability and safety outcomes of clinical interest occurring at any time during 3-month double-blind treatment, including AEs and discontinuation due to an AE, were assessed for daridorexant. Data were extracted by study arm from published literature.18 The population (denominator) was the number of all randomized participants who received ≥ 1 dose of study drug. Tolerability and safety outcomes of clinical interest have been previously extracted and reported for lemborexant16 through month 1 (SUNRISE 1 and SUNRISE 2) and month 3 (SUNRISE 2).
Data Analysis
NNT and NNH, with their respective 95% CIs, were calculated for daridorexant versus placebo and lemborexant versus placebo in each individual study. Study data were also pooled as appropriate. The daridorexant 10-mg dose group was not included in analyses or in dose group pooling as it is not an approved dose. LHH was calculated to illustrate potential trade-offs for efficacy (response) and tolerability outcomes (commonly encountered AEs).
In this analysis, the use of the terms statistically significant and non–statistically significant is descriptive rather than inferential. In all instances, if the 95% CI included “infinity,” the result is considered not statistically significant at the P < .05 threshold. The notation “NS” (ie, not significant) is used rather than showing the non-continuous 95% CIs generated when statistical significance is not achieved. In general, a NNT versus placebo < 10 is considered to be a clinically relevant effect size difference, with a NNT < 5 being considered even more desirable.14,15 A NNH versus placebo > 10 is generally considered acceptable, with a NNH > 20 deemed more desirable. A negative value for a NNH versus placebo can occur when an AE is more common with placebo than with study drug. In this instance, “no difference” (ND) has been noted to avoid interpreting such difference as a tolerability advantage over placebo. Because a meaningful LHH cannot be calculated with a zero or negative NNH value, for negative NNH estimates, a NNH of 1,000 was used as an approximation for LHH calculations.23,24 A LHH > 1 indicates that a treatment is more likely to help than harm.14
Formulae Used
The absolute risk increase (ARI) was calculated as the incidence on medication (f1) less the incidence on placebo (f2) (ARI = f1 – f2). The corresponding 95% CI was calculated by the equations below, for which z = 1.96 for a 95% CI.
Lower bound of the CI = `ARI-z√((f_1 (1-f_1))/n_1 +(f_2 (1-f_2))/n_2 )`
Upper bound of the CI = `ARI+z√((f_1 (1-f_1))/n_1 +(f_2 (1-f_2))/n_2 )`
NNT or NNH were calculated as the reciprocal of ARI (1/ARI) and rounded up to the next highest whole number. The 95% CI for the NNT or NNH was calculated by taking the reciprocal of the lower and upper bounds of the CI for the ARI. LHH was calculated as the ratio of NNH to NNT (LHH = NNH/NNT).
RESULTS
Lemborexant
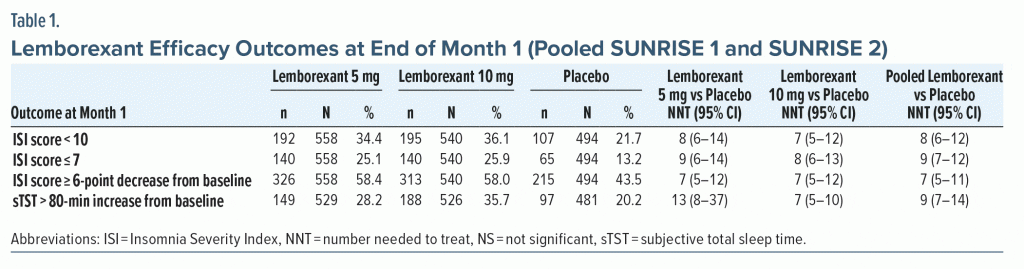
Efficacy—NNT. The categorical efficacy outcomes at the end of the SUNRISE 1 study at month 1 demonstrated a statistically significantly higher responder rate with either lemborexant 5 mg or 10 mg compared with placebo for a variety of response definitions: ISI score < 10, ISI score ≤ 7, ISI score ≥ 6-point decrease from baseline, and sTST > 80-minute increase from baseline (Supplementary Table 2). Significant improvements were also observed for PSG response of ≥ 50% LPS or WASO improvement from baseline for both lemborexant doses and ≥ 75% LPS or WASO improvement for lemborexant 10 mg (Supplementary Table 2). Most NNT values versus placebo for lemborexant were ≤ 10, with the most robust NNT being 5 for ≥ 50% WASO improvement (5 mg and 10 mg) and sTST improvement > 80 minutes (10 mg). A negative NNT for the active control, zolpidem ER 6.25 mg, for LPS outcomes indicates placebo outperformed zolpidem ER on these measures. Efficacy results for SUNRISE 2 showed similar trends of significance of NNT for both lemborexant doses versus placebo for ISI measures, with generally more robust NNT estimates at month 3 compared with month 1 (Supplementary Table 3).
In pooled efficacy data from SUNRISE 1 and SUNRISE 2, NNT values versus placebo at the end of month 1 for pooled lemborexant 5-mg and 10-mg dose groups were < 10 for all relevant outcomes: ISI score < 10, ISI score ≤ 7, ISI score ≥ 6-point decrease from baseline, and sTST > 80-minute increase from baseline (Table 1). There was a potential dose response for the sTST outcome (NNT vs placebo: lemborexant 10 mg, 7; lemborexant 5 mg, 13) with a NNT for the lemborexant 10-mg group versus 5-mg group of 14 (95% CI, 8–51).
Tolerability—NNH. In pooled tolerability analysis of SUNRISE 1 and SUNRISE 2 through month 1 (Table 2),16,19,20 there were no NNH values versus placebo for lemborexant that were < 10. The lowest significant NNH estimates reported for pooled lemborexant 5-mg and 10-mg doses were for somnolence (19) and fatigue (56). The NNH for somnolence (lemborexant 10 mg, 15; lemborexant 5 mg, 28) indicated a potential dose response (NNH for somnolence in lemborexant 10-mg group vs 5-mg group, 30 [95% CI, 16–182]). Rates of discontinuation due to any AE, including somnolence, were low and not statistically significant through month 1, with NNH values versus placebo of approximately 100 or higher for these outcomes regardless of dose (Table 2). Month 3 tolerability outcomes were available only in SUNRISE 2 (Table 2). At month 3 for commonly reported AEs (≥ 2% in any group) there were no NNH values < 10. As at month 1, significant NNHs for pooled 5-mg and 10-mg lemborexant doses were lowest for somnolence (12) and fatigue (29), with a potential dose response for somnolence (lemborexant 10 mg, 10; lemborexant 5 mg, 15; NNH for somnolence in lemborexant 10-mg group vs 5-mg group, 27 [NS]).
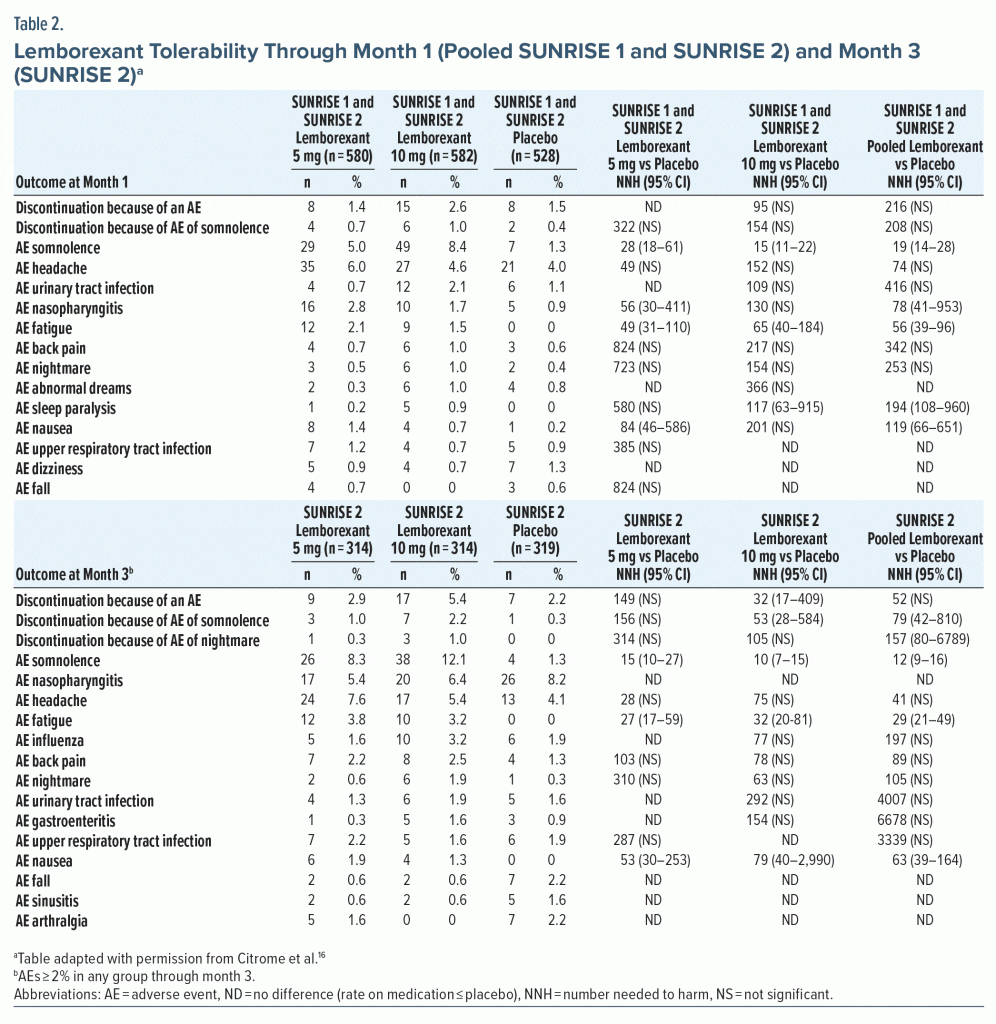
LHH. Calculating the LHH for discontinuation due to an AE for pooled lemborexant 5-mg and 10-mg doses versus placebo at month 1 (NNH = 216 [NS]) using the range of statistically significant NNTs versus placebo (5–14) equated to a LHH range of 15.4–43.2. At month 3 (NNH = 52 [NS]; NNT range, 5–10), the LHH range was 5.2–10.4. As there were fewer discontinuations due to an AE in the lemborexant 5-mg group compared with the 10-mg group, the LHH range for the 5-mg group alone was more advantageous (LHH range for discontinuation due to an AE: month 1, 76.9–200; month 3, 13.5–298). The month 3 LHH with pooled lemborexant 5-mg and 10-mg doses for an AE of fatigue (NNH = 29) or an AE of somnolence (NNH = 12) versus ISI score ≥ 6-point decrease from baseline (NNT = 5) or sTST > 80-minute increase (NNT = 10) ranged from 1.2 to 5.8.
Daridorexant
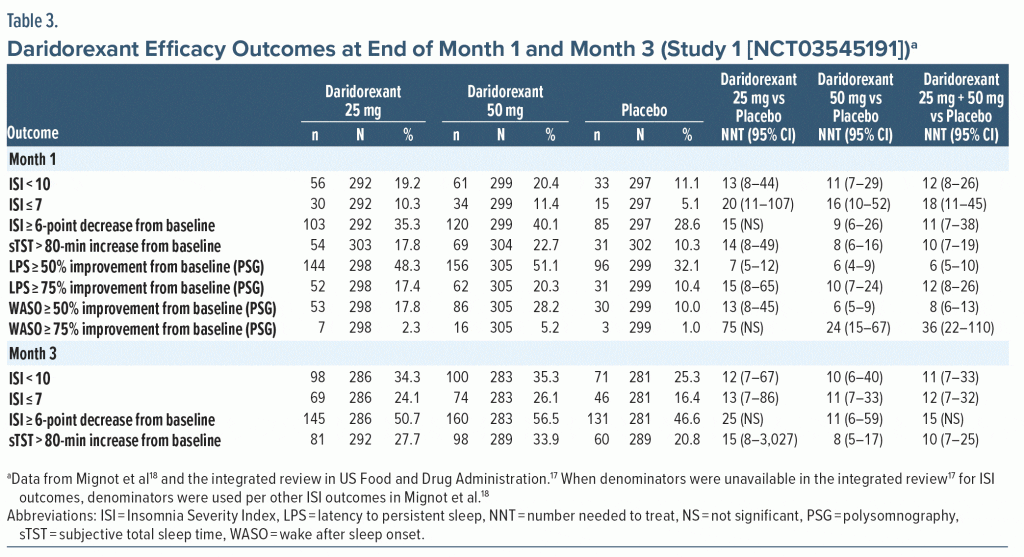
Efficacy—NNT. The categorical efficacy outcomes for Study 1 at the end of month 1 and month 3 (Table 3) demonstrated consistently statistically significant NNT values versus placebo for daridorexant 50 mg for a variety of response definitions including ISI score < 10, ISI score ≤ 7, ISI score ≥ 6-point decrease from baseline, and sTST improved by > 80 minutes from baseline. NNT estimates were not statistically significant for daridorexant 25 mg for ISI score ≥ 6-point decrease from baseline, suggesting a clinically relevant dose response. Additionally, the NNT versus placebo was numerically more robust for daridorexant 50 mg compared with daridorexant 25 mg at either month 1 or month 3 for all efficacy outcomes (PSG outcomes were reported only at month 1; Table 3). NNT estimates that were < 10 in Study 1 for daridorexant 50 mg were ISI score ≥ 6-point decrease from baseline at month 1, sTST > 80-minute increase from baseline at month 1 and month 3, WASO ≥ 50% improvement from baseline at month 1, and LPS ≥ 50% improvement from baseline at month 1. The 25-mg daridorexant group also had a NNT < 10 for LPS ≥ 50% improvement from baseline at month 1. NNT estimates for ISI score ≤ 7 were more robust at month 3 than at month 1. For daridorexant Study 2 (Supplementary Table 4), no NNT values versus placebo were < 10 for the daridorexant 25-mg dose group. NNT values versus placebo were statistically significant for daridorexant 25 mg for ISI score < 10 and sTST > 80-minute increase from baseline. NNT estimates were generally more robust at month 3 than at month 1.
Pooled efficacy data from daridorexant Study 1 and Study 2 are shown in Supplementary Table 5 and do not include the 10-mg dose group, as it is not commercially available and is considered subtherapeutic. For pooled doses of 25 mg and 50 mg, no NNT values versus placebo were < 10. Estimates were more robust at month 3 than at month 1 for ISI score < 10 and sTST > 80-minute increase from baseline.
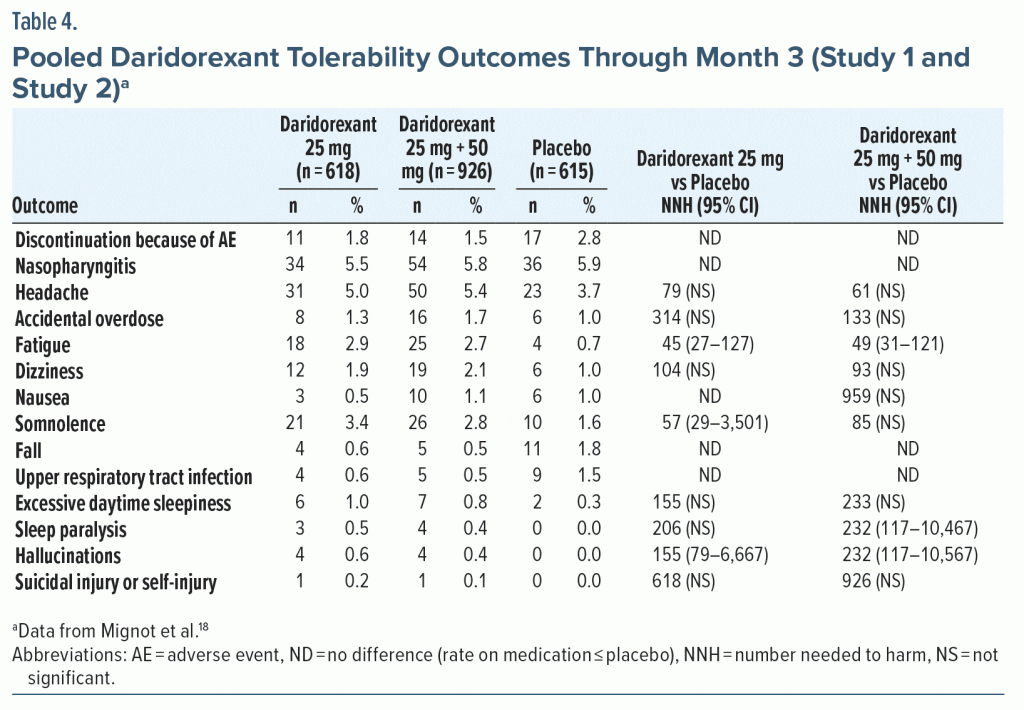
Tolerability—NNH. Rates for discontinuation because of an AE were higher for the placebo groups than for any of the daridorexant dose groups in Study 1 (Supplementary Table 6) or Study 2 (Supplementary Table 7). Hence, the NNH values versus placebo for this outcome were negative numbers. In Study 1, all NNH estimates for AEs were > 10; for the non-pooled daridorexant dose groups, the NHH estimates versus placebo were not statistically significant. In the pooled 25-mg and 50-mg dose groups, AEs of fatigue (NNH = 62) and dizziness (NNH = 69) were statistically significant, but the NNH values versus placebo were weak in terms of effect size (ie, much greater than 10; see Supplementary Table 6). The AE of somnolence was not statistically significant (NNH = 155). In Study 2, all NNH estimates were > 10 (Supplementary Table 7). A statistically significant NNH value versus placebo was reported for fatigue for daridorexant 25 mg (NNH = 35). The NNH for an AE of somnolence was not significant for daridorexant 25 mg (NNH = 52). In pooled 25-mg and 50-mg dose groups from Study 1 and Study 2 (Table 4), all NNH estimates were > 10; the most robust statistically significant NNH versus placebo was reported for fatigue (NNH = 49), and the NNH for an AE of somnolence (NNH = 85) was not significant.
LHH. Comparing the NNH versus placebo for discontinuation due to an AE for pooled daridorexant 25-mg and 50-mg doses (imputed to be 1,000) to the range of statistically significant NNTs versus placebo (10–13) equates to a LHH range of 76.9–100 at month 3. Efficacy outcomes were more robust for the daridorexant 50-mg group alone, with statistically significant NNT estimates versus placebo of 8–11 (excluding ISI score < 22), resulting in a LHH at month 3 ranging from 90.9 to 125. The month 3 LHH for the pooled daridorexant 25-mg and 50-mg doses for an AE of fatigue (NNH = 49) or an AE of somnolence (NNH = 85) versus ISI score ≥ 6-point decrease from baseline (NNT = 13) or sTST > 80-minute increase (NNT = 10) ranged from 3.8 to 8.5.
Indirect Comparisons
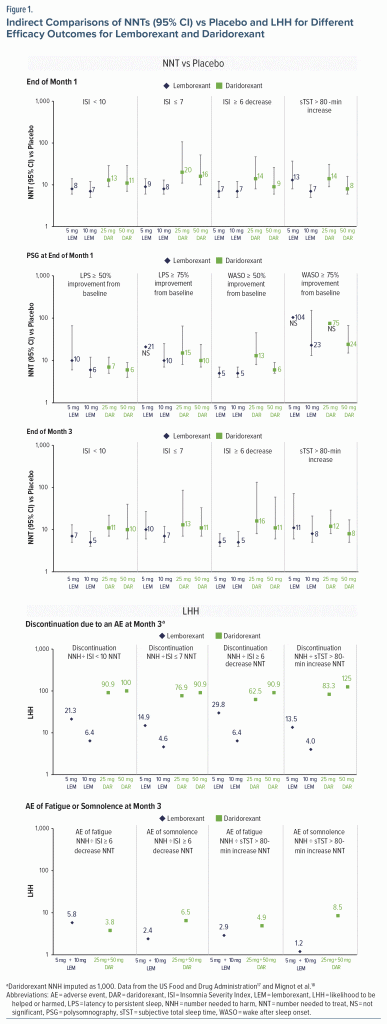
The efficacy outcome NNT estimates for ISI and sTST for the available commercial dose strengths of lemborexant and daridorexant are compared in Figure 1. For most outcomes, the NNT effect sizes were more robust for lemborexant than for daridorexant at month 1 and month 3.
PSG measures were available for comparison only at month 1 and indicated similar NNT ranges for lemborexant and daridorexant for LPS improvement of ≥ 50% or ≥ 75% from baseline (Figure 1). NNT estimates for WASO improvement of ≥ 50% from baseline were more robust for lemborexant than for daridorexant, whereas NNT estimates for WASO improvement of ≥ 75% demonstrated small effect size differences from placebo (Figure 1).
For lemborexant, the discontinuation rates due to an AE through month 3 (data source: SUNRISE 2) were higher than for placebo (Table 2); the NNH was quantifiable but not statistically significant. For the pooled daridorexant studies, the discontinuation rates due to an AE through month 3 were higher for placebo than for daridorexant, yielding an imputed NNH of 1,000 that was used for the LHH calculations (Table 4). These differences in discontinuation rates due to an AE resulted in more favorable LHH calculations for daridorexant than for lemborexant despite less robust NNT for efficacy outcomes for daridorexant (Figure 1). The 3-month NNH for pooled daridorexant doses (Study 1 and 2 pooled) was more favorable than that for the pooled lemborexant doses (in SUNRISE 2) for AEs of fatigue (pooled daridorexant = 49; pooled lemborexant = 29) and somnolence (pooled daridorexant = 85; pooled lemborexant = 12). The LHH values contrasting efficacy (ISI score ≥ 6-point decrease from baseline and sTST > 80-minute increase from baseline) and tolerability (AEs of somnolence and fatigue) were > 1 for both daridorexant and lemborexant (Figure 1).
DISCUSSION
The goal of an insomnia medication is to allow patients to fall asleep, stay asleep, wake, and function well.6 For insomnia medications, some of the key pharmacokinetic properties that may impact efficacy (eg, onset and maintenance) and safety (eg, duration of action and next day effects) may include absorption, receptor interaction, half-life curve, and endogenous orexin levels (which are highest upon awakening25). Each DORA has unique characteristics in terms of absorption, time to receptor occupancy (Kon and Koff), time to onset of action, half-life, and accumulation at steady state10-12; these pharmacokinetic profiles are published elsewhere.26,27 However, the clinical impacts of these different characteristics have not been tested, and head-to-head studies of DORAs have not been conducted. As such, this analysis to indirectly compare clinical trial data of lemborexant and daridorexant using NNT, NNH, and LHH assessments is relevant to aid clinical decision-making.
A NNT of < 10 is desirable and clinically relevant, as it indicates that fewer than 10 patients would require treatment to observe a benefit compared with placebo.14 A NNT of < 10 was observed more consistently with lemborexant than with daridorexant for subjectively reported ISI and sTST measures, indicating a more robust clinical effect with lemborexant. Larger effect sizes were reported for lemborexant than for daridorexant at month 1 and month 3, with the most robust improvements at month 3. In a previous report comparing hypnotics for insomnia treatment, the NNT estimates for different categorical efficacy outcomes were also generally < 10 at month 1 and month 3 (when available) for lemborexant (5 mg or 10 mg), zolpidem ER (6.25 mg or 12.5 mg), doxepin (3 mg or 6 mg), and eszopiclone (2 mg).16 The most robust NNT effect sizes (NNT = 5) were reported for ISI score ≥ 6-point decrease for all doses of lemborexant at month 3 and for ISI score < 10 for lemborexant 10 mg. Categorial data regarding an ISI score ≥ 6-point decrease are also available for suvorexant.16 NNT estimates for an ISI ≥ 6-point decrease from baseline with suvorexant 15 mg or 20 mg at month 1 (10 [95% CI, 7–19]) and month 3 (8 [95% CI, 6–14]) were intermediate between the NNT ranges for pooled lemborexant doses (5–7) and pooled daridorexant doses (12–13). For PSG outcomes, only available for comparison at month 1, NNT estimates for lemborexant were similar to those for daridorexant. For many of the efficacy outcomes, the 95% CIs for lemborexant and daridorexant overlapped, indicating an appropriately designed trial including both treatments would be needed to more precisely compare their efficacy profiles.
Generally, a NNH > 10 is desirable, although a NNH < 10 may still be accepted for an AE that is mild, is temporary, does not result in discontinuation, does not pose a serious health risk, or causes minimal distress.14 Both lemborexant and daridorexant had NNH ≥ 10 indicative of a favorable tolerability profile compared with placebo. At month 3, rates of discontinuation due to an AE were not significant for either pooled lemborexant doses or pooled daridorexant doses, with higher discontinuation rates with placebo than observed with daridorexant. NNH versus placebo for both fatigue and somnolence favored daridorexant over lemborexant. NNH versus placebo for somnolence with daridorexant was also more favorable than those reported previously for other hypnotics (except ramelteon 8 mg).16 Somnolence with lemborexant was dose-dependent, and the prescribing information for lemborexant recommends a dose of lemborexant 5 mg, to be increased up to 10 mg based on clinical response and tolerability.11 Nonetheless, rates of discontinuation due to an AE of somnolence were low with lemborexant.
LHH ratios may help place NNH and NNT in a broader context and may reflect instances in which a lower NNH may be acceptable if a treatment has a desirable NNT (< 10). A LHH of much greater than 1 was observed for discontinuation due to an AE at month 3 for both lemborexant and daridorexant, with higher LHH reported with daridorexant across the subjectively reported outcomes. LHHs for sTST > 80-minute increase and ISI ≥ 6-point decrease from baseline were > 1 for both daridorexant and lemborexant for AEs of fatigue and somnolence, indicating the treatments are more likely to be efficacious than cause these AEs.
A limitation of this analysis includes the potential for introduced biases. Indirect comparisons can be biased by heterogeneity in study design between trials that may include differences in baseline characteristics, outcome definitions, medication dosing, trial duration, and timing of assessments.28 Trials included in this analysis had different study designs for lemborexant and daridorexant and the enrollment of similar yet different populations (Supplementary Table 1). For example, the lemborexant SUNRISE 1 study was limited to older adults, ≥ 55 years old, with an ISI score ≥ 13, while the other 3 studies were open to adults ≥ 18 years old with an ISI score ≥ 15. In SUNRISE 1, participants were not required to have sleep onset difficulties, whereas the daridorexant studies were limited to patients with a history of sSOL ≥ 30 minutes. Moreover, the mean baseline LPS in SUNRISE 1 (range, 44.6–44.9 minutes) was shorter than the mean baseline LPS in studies of daridorexant (range, 63.6–71.8 min). Despite this population difference, the NNT for LPS ≥ 50% improvement at month 1 with pooled lemborexant was still significant in SUNRISE 1 (Supplementary Table 2). Population differences also exist regarding ethnic diversity, with the lemborexant SUNRISE 1 and SUNRISE 2 studies including 25.4% and 28.5% non-White participants, respectively, compared with 10%–12% for daridorexant studies 1 and 2. Additionally, objective PSG measures to confirm severity for study inclusion were not required for all of the trials. These variabilities in study design limit the ability to make definitive statements based on the results of these analyses. Further research using direct head-to-head clinical trials among DORAs would be beneficial to provide more robust evidence for the relative efficacy and safety of lemborexant and daridorexant.
An additional limitation is that the data from this study were limited to categorical outcomes that were reported previously in the literature and regulatory documents for daridorexant. For example, data for subjective WASO and sSOL were not available for daridorexant, thereby preventing comparison. As available dichotomous data for suvorexant differed, it was not possible to conduct an in-depth comparison of all 3 FDA-approved DORAs. As is commonly a concern when interpreting clinical trial data for daily practice, results may not be generalizable to the real-world treatment population due to the strict inclusion and exclusion criteria of clinical studies, which often exclude patients with comorbidities. NNH versus placebo for discontinuation specifically may not always generalize to overall tolerability, as the reasons for clinical trial discontinuation are complex. Additionally, due to the short-term duration and small sample sizes of many trials, the NNH for delayed adverse outcomes or uncommon AEs were likely not captured. Lastly, not all AEs leading to discontinuation are associated with study drug. Generalizability to the real-world population is further limited by the short duration of some of the trials included in this analysis, which did not capture long-term outcomes. As long-term data for insomnia treatments were not required by regulatory agencies until recent years, the majority of clinical trials for insomnia treatments do not report long-term outcomes.29 While the SUNRISE 2 study investigated long-term lemborexant treatment for up to 12 months (6 months placebo-controlled), future long-term trials would be required to characterize and compare the long-term safety and efficacy of lemborexant and daridorexant.
CONCLUSION
This analysis supports the use of lemborexant and daridorexant for the treatment of adults with insomnia, with a more favorable NNT efficacy profile for lemborexant and more favorable NNH estimates for discontinuation due to an AE for daridorexant. For both agents, NNH estimates for discontinuation due to an AE were not significant. The LHH for an AE of somnolence indicated a tolerability advantage for daridorexant. Definitive statements on relative benefits between the 2 treatments, however, should be made with caution. Well-designed direct head-to-head clinical trials among the DORAs would be helpful to further characterize any potential differences in their efficacy and safety profiles.
Article Information
Published Online: October 2, 2023. https://doi.org/10.4088/JCP.23m14851
© 2023 Physicians Postgraduate Press, Inc.
Submitted: March 8, 2023; accepted July 28, 2023.
To Cite: Citrome L, Juday TR, Lundwall C. Lemborexant and daridorexant for the treatment of insomnia: an indirect comparison using number needed to treat, number needed to harm, and likelihood to be helped or harmed. J Clin Psychiatry. 2023;84(6):23m14851.
Author Affiliations: Department of Psychiatry and Behavioral Sciences, New York Medical College, Valhalla, New York (Citrome); Eisai Inc., Nutley, New Jersey (Juday, Lundwall).
Corresponding Author: Leslie Citrome, MD, MPH, 11 Medical Park Drive, Suite 102, Pomona, NY 10970 (nntman@gmail.com).
Relevant Financial Relationships: Dr Citrome serves as consultant to AbbVie/Allergan, Acadia, Adamas, Alkermes, Angelini, Astellas, Avanir, Axsome, BioXcel, Boehringer Ingelheim, Cadent Therapeutics, Cerevel, Clinilabs, COMPASS, Eisai, Enteris BioPharma, HLS Therapeutics, Idorsia, INmune Bio, Impel, Intra-Cellular Therapies Inc., Janssen, Karuna, Lundbeck, Lyndra, Medavante-ProPhase, Marvin, Merck, Neurocrine, Neurelis, Novartis, Noven, Otsuka, Ovid, Praxis, Recordati, Relmada, Reviva, Sage, Sunovion, Supernus, Teva, and University of Arizona, and has done one-off ad hoc consulting for individuals/entities conducting marketing, commercial, or scientific scoping research; has served as speaker for AbbVie/Allergan, Acadia, Alkermes, Angelini, Axsome, BioXcel, Eisai, Idorsia, Intra-Cellular Therapies Inc., Janssen, Lundbeck, Neurocrine, Noven, Otsuka, Recordati, Sage, Sunovion, Takeda, Teva, and CME activities organized by medical education companies such as Medscape, NACCME, NEI, Vindico, and universities and professional organizations/societies; owns stocks (small number of shares of common stock) for Bristol-Myers Squibb, Eli Lilly, Johnson & Johnson, Merck, Pfizer purchased > 10 years ago and stock options for Reviva; and receives royalties/publishing income from Taylor & Francis (Editor-in-Chief, Current Medical Research and Opinion, 2022–date), Wiley (Editor-in-Chief, International Journal of Clinical Practice, through end 2019), UpToDate (reviewer), Springer Healthcare (book), and Elsevier (Topic Editor, Psychiatry, Clinical Therapeutics). Drs Juday and Lundwall are employees of Eisai Inc.
Funding/Support: This work was funded by Eisai Inc. (Nutley, New Jersey, USA). Eisai Inc. produces lemborexant (Dayvigo).
Role of the Funders/Sponsors: Although employees of Eisai Inc. reviewed the manuscript, final decisions on content and submission of the manuscript were at the sole discretion of the lead author, Dr Citrome.
Previous Presentation: Poster presented at Psych Congress; September 17–20, 2022; New Orleans, Louisiana • 2022 NEI Congress; November 3–6, 2022; Colorado Springs, Colorado.
Acknowledgements: Sarah Engelberth, PhD, of Medical Expressions (Chicago, Illinois) provided editorial support for the manuscript, which was funded by Eisai, Inc.
Additional Information: All data for this study were obtained from the published literature and publicly available regulatory documents, except for a small number of data elements from the lemborexant clinical trial database. The latter is proprietary and not currently freely available.
ORCID: Leslie Citrome: https://orcid.org/0000-0002-6098-9266; Timothy R. Juday: https://orcid.org/0000-0002-7310-4113; Christie Lundwall: https://orcid.org/0000-0002-5524-0752
Supplementary Material: Available at Psychiatrist.com.
CLINICAL POINTS
- Many pharmacologic and nonpharmacologic options are available to treat insomnia, a common and chronic condition, and each option has associated benefits and risks.
- Using number needed to treat and number needed to harm analyses may help place new insomnia medications into clinical perspective with other treatment options.
Quick Links: