This work may not be copied, distributed, displayed, published, reproduced, transmitted, modified, posted, sold, licensed, or used for commercial purposes. By downloading this file, you are agreeing to the publisher’s Terms & Conditions.
Original Research
Earlier Use of Long-Acting Injectable Paliperidone Palmitate Versus Oral Antipsychotics in Patients With Schizophrenia: An Integrated Patient-Level Post Hoc Analysis
Published: September 25, 2023
ABSTRACT
Objective: To assess the efficacy and safety of paliperidone palmitate (PP) long-acting injectable antipsychotic (LAI) versus oral antipsychotic (OAP) treatment in adult patients diagnosed with schizophrenia (per DSM-IV or DSM-5 criteria) and varying duration of illness (0–3 years and > 3 years).
Methods: Patient-level data from the PRIDE, PROSIPAL, and DREaM studies were included in a post hoc analysis. Efficacy and safety outcomes, including relapse assessments, Personal and Social Performance scale scores, Medication Satisfaction Questionnaire total scores, and treatment-emergent adverse events (TEAEs), were measured systematically. Treatment failure (TF) included relapses due to psychiatric hospitalizations, arrest or incarceration, suicidal or homicidal ideation or behavior, discontinuation due to inadequate efficacy and/or safety or tolerability, treatment supplementation due to inadequate efficacy, and significant worsening of symptoms.
Results: Fewer TFs were observed with PP versus OAP in both the 0–3 years group (17.7% and 25.3%, respectively) and the > 3 years group (32.3% and 42.4%, respectively). Time to first TF was significantly longer with PP versus OAP treatment in both duration of illness groups. TEAE rates were similar between the PP and OAP groups, with no new safety signals identified.
Conclusions: This post hoc analysis suggests that PP provides significant benefit in reducing TF or relapse compared with OAPs regardless of duration of illness and highlights the potential benefit of implementing PP earlier in the course of schizophrenia.
Trial Registration: ClinicalTrials.gov identifiers NCT01157351 (PRIDE), NCT01081769 (PROSIPAL), and NCT02431702 (DREaM)
J Clin Psychiatry 2023;84(6):23m14788
Author affiliations are listed at the end of this article.
Schizophrenia is a chronic mental illness associated with a wide array of symptoms, including hallucinations, delusions, impaired cognition, decreased motivation, diminished interests, and emotional blunting. Individuals with schizophrenia often struggle with consistent medication adherence, which can result in poor outcomes, including relapse and suicidal behavior or ideation.1 Early intervention in schizophrenia is critical because with each subsequent relapse, there is further functional deterioration, decreased response to treatment, and worsening of clinical outcomes.2,3 Schizophrenia may also cause a high degree of disability, accounting for 1.1% of the total disability-adjusted life-years (DALYs), and was listed as the eighth leading cause of DALYs worldwide in individuals 15–44 years of age.4 In addition to poor clinical outcomes, there is a tremendous economic cost associated with schizophrenia.5 In 2020, the estimated economic burden in the United States was $281.6 billion.6 Two-thirds of patients with schizophrenia who are treated with oral antipsychotics (OAPs) are nonadherent.7 Relapse, often related to poor treatment adherence, has been associated with complications and negative health events such as hospitalization and other disruptions to life (eg, dropping out of school, loss of employment) that make recovery especially challenging. Nonadherence to antipsychotic medication and subsequent relapse resulting in hospitalization represent a substantial proportion of overall health care costs among patients with schizophrenia.8
The majority of adult patients with schizophrenia begin treatment with an OAP before considering other treatment options; however, long-acting injectable antipsychotics (LAIs) have the potential to improve adherence and long-term health outcomes.9–13 Although emerging evidence suggests that LAIs reduce the risk of treatment failure (TF) or relapse in adult patients recently diagnosed with schizophrenia (ie, those with shorter duration of illness),11,14,15 they are typically reserved for patients with a long history of schizophrenia.11,14–16 Previous studies have demonstrated the benefit of initiating treatment with LAIs in patients who were recently diagnosed with schizophrenia (≤ 3 years). This aligns with the “critical period” concept, which posits that there is a rapid progression of illness within the first 3–5 years, during which therapeutic intervention has the largest impact. Several national organizations have provided guidance supporting the use of LAIs in patients recently diagnosed with schizophrenia; however, both health care providers and patients can be reluctant to consider LAIs as a treatment option.16–18
Given the potential benefits of LAIs in adult patients recently diagnosed with schizophrenia and the updated recommendations from clinical practice guidelines, it is of clinical interest to examine the advantages of LAI use in this population.11,19,20 Paliperidone palmitate (PP), an LAI indicated for the treatment of adults with schizophrenia, is available in once-monthly (PP1M),21 once-every-3-months (PP3M, indicated for patients who have been adequately treated with PP1M for ≥ 4 months),22 and once-every-6-months (PP6M, indicated for patients who have been adequately treated with PP1M for ≥ 4 months or who have received ≥ 1 PP3M 3-month cycle)23 formulations. PP has been shown to be effective in maintaining management of symptoms and delaying time to relapse in patients with schizophrenia.24–26 A post hoc analysis was utilized to assess the efficacy and safety of PP LAI versus OAP treatment in adult patients diagnosed with schizophrenia and varying duration of illness (0–3 years and > 3 years).
METHODS
Study Selection
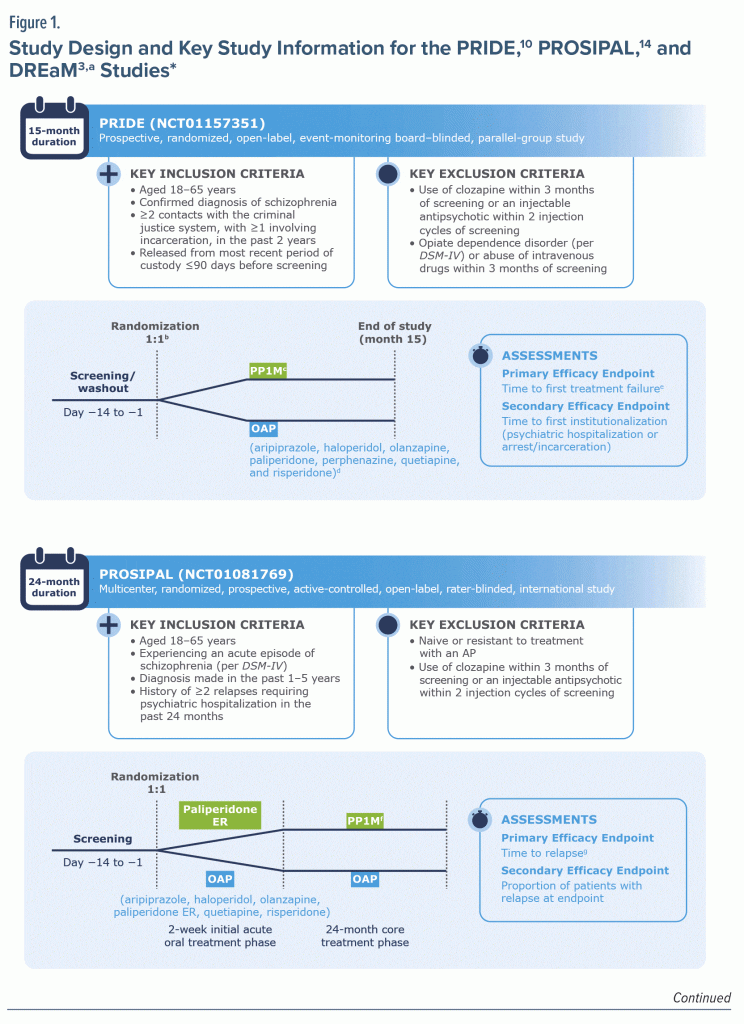
Three studies were included in the post hoc analysis: PRIDE (NCT01157351),10 PROSIPAL (NCT01081769),14 and DREaM (NCT02431702).3 Because all 3 studies were Janssen-sponsored, the authors had access to patient-level data. The availability of patient-level data (ie, raw data for each individual patient in the study) for all 3 studies allowed the post hoc analysis to preserve clustering of patients within studies and summarize the evidence on a particular clinical question from multiple related studies, such as treatment effectiveness (Figure 1). These studies were also appropriate for the post hoc analysis because they had similar inclusion and exclusion criteria as well as similar definitions for relapse or TF. Only patients with schizophrenia were included in the analysis; patients in the DREaM study with a diagnosis of schizophreniform disorder were excluded.
Outcomes
Efficacy and safety outcomes, including relapse assessments, Personal and Social Performance (PSP) scale27 scores, Medication Satisfaction Questionnaire (MSQ)28 total scores, and treatment-emergent adverse events (TEAEs) were measured systematically. Across studies, the definition of TF included relapses due to psychiatric hospitalizations, arrest or incarceration, suicidal or homicidal ideation or behavior, discontinuation due to inadequate efficacy and/or safety or tolerability, treatment supplementation due to inadequate efficacy, and significant worsening of symptoms (based on the Positive and Negative Syndrome Scale29 or the Clinical Global Impressions Scale30) (Figure 1).
Social functioning was assessed with the PSP scale, a 100-point, single-item rating scale that is subdivided into ten 10-point intervals.27 The ratings assess the patient’s functioning in 4 areas: (1) socially useful activities, (2) personal and social relationships, (3) self-care, and (4) disturbing and aggressive behaviors. Patient satisfaction antipsychotic medication was evaluated using the MSQ.28 This questionnaire consists of a 1-item global patient-rated scale in which patients were asked to respond on a 7-point scale, ranging from extremely dissatisfied (1) to extremely with satisfied (7) to the following statement: “The way you feel about taking your study medication is ____.”
Data Analysis
The included studies had different cutoffs for illness duration (DREaM: < 2 years; PROSIPAL: 1–5 years; PRIDE: ≤ 5 years and > 5 years); however, all 3 studies had individual patient-level data available, allowing cutoffs of 0–3 years and > 3 years to be used. An additional sensitivity analysis using cutoffs of 2 and 5 years was also performed. Cumulative distribution functions of time to first TF (TtFTF) or relapse were estimated using the Kaplan-Meier method. Group differences were examined using a log rank test. Hazard ratios (HRs) and 95% confidence intervals (CIs) for between-group differences in risk of TF or relapse were based on a Cox proportional hazards model. Proportional hazard assumptions for each comparison were verified using both graphical diagnostic and statistical testing. Group differences in change scores from baseline to endpoint (month 15) for the PSP total score were examined using mixed-model repeated measures with factors for treatment, study ID, visit, and treatment-by-visit interaction as fixed effects. Baseline PSP score was used as a covariate. Within-subject correlation of the repeated measures was modeled using an unstructured covariance matrix after examination of model fits for other type of covariance structures. Changes in MSQ score were examined using an analysis of covariance (ANCOVA) model because only one endpoint assessment was captured after baseline. The ANCOVA model included factors for treatment, study ID, and baseline MSQ score. TEAEs and serious adverse events (SAEs) were documented and are summarized descriptively.
RESULTS
Baseline Demographics and Disease Characteristics
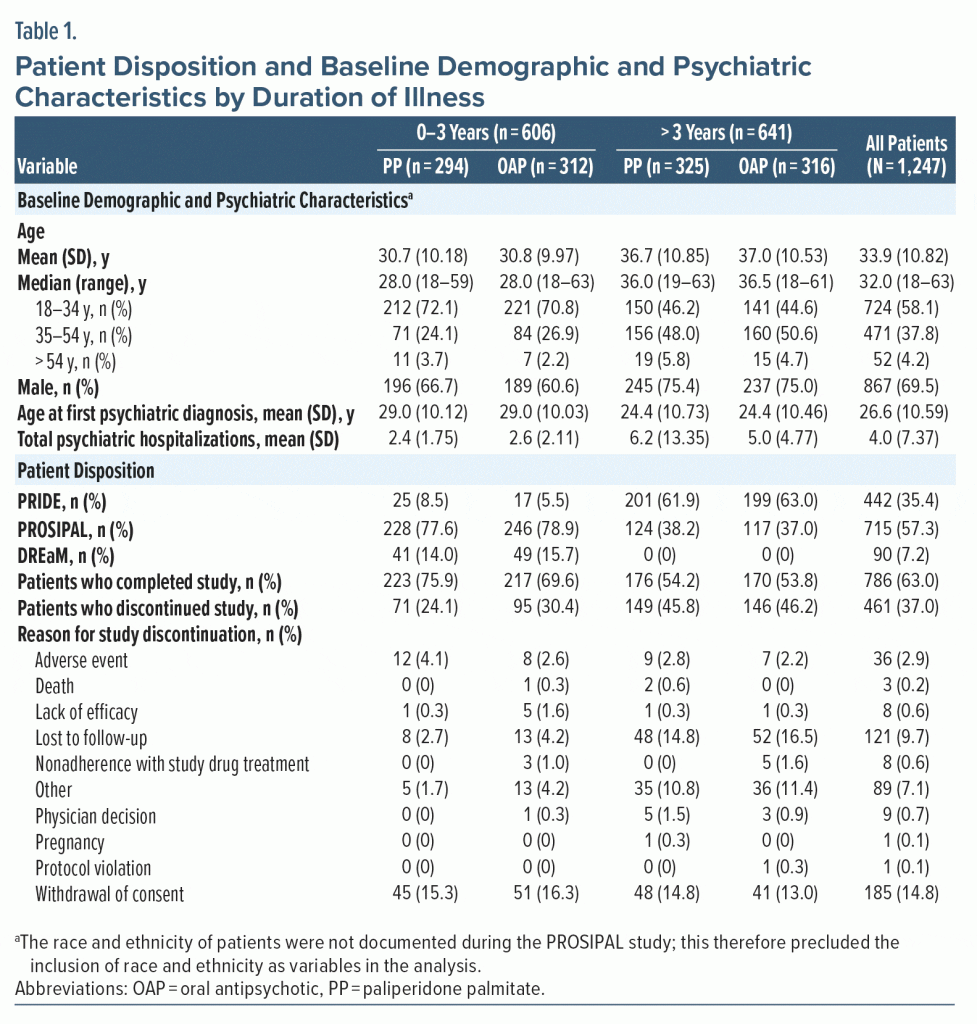
A sample of 1,247 patients were included in the intention-to-treat analysis set (0–3 years: PP, n = 294, OAP, n = 312; > 3 years: PP, n = 325, OAP, n = 316); most patients were male (69.5%), and the mean age was 33.9 years (Table 1). The mean (SD) age of first psychiatric diagnosis was 29.0 (10.12) and 29.0 (10.03) years in the 0–3 years PP and OAP groups, respectively, and 24.4 (10.73) and 24.4 (10.46) years in the > 3 years PP and OAP groups, respectively. Mean [SD] total psychiatric hospitalizations were higher in the > 3 years group (PP: 6.2 [13.35]; OAP: 5.0 [4.77]) versus the 0–3 years group (PP: 2.4 [1.75]; OAP: 2.6 [2.11]).
Patient Disposition
In the 0–3 years group, 71 patients (24.1%) and 95 patients (30.4%) discontinued the study in the PP and OAP groups, respectively; in the > 3 years group, 149 patients (45.8%) and 146 patients (46.2%) discontinued the study in the PP and OAP groups, respectively. The most common reasons for discontinuation are listed in Table 1.
Efficacy Outcomes
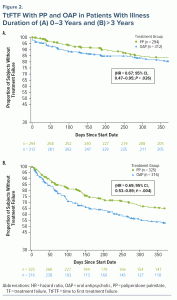
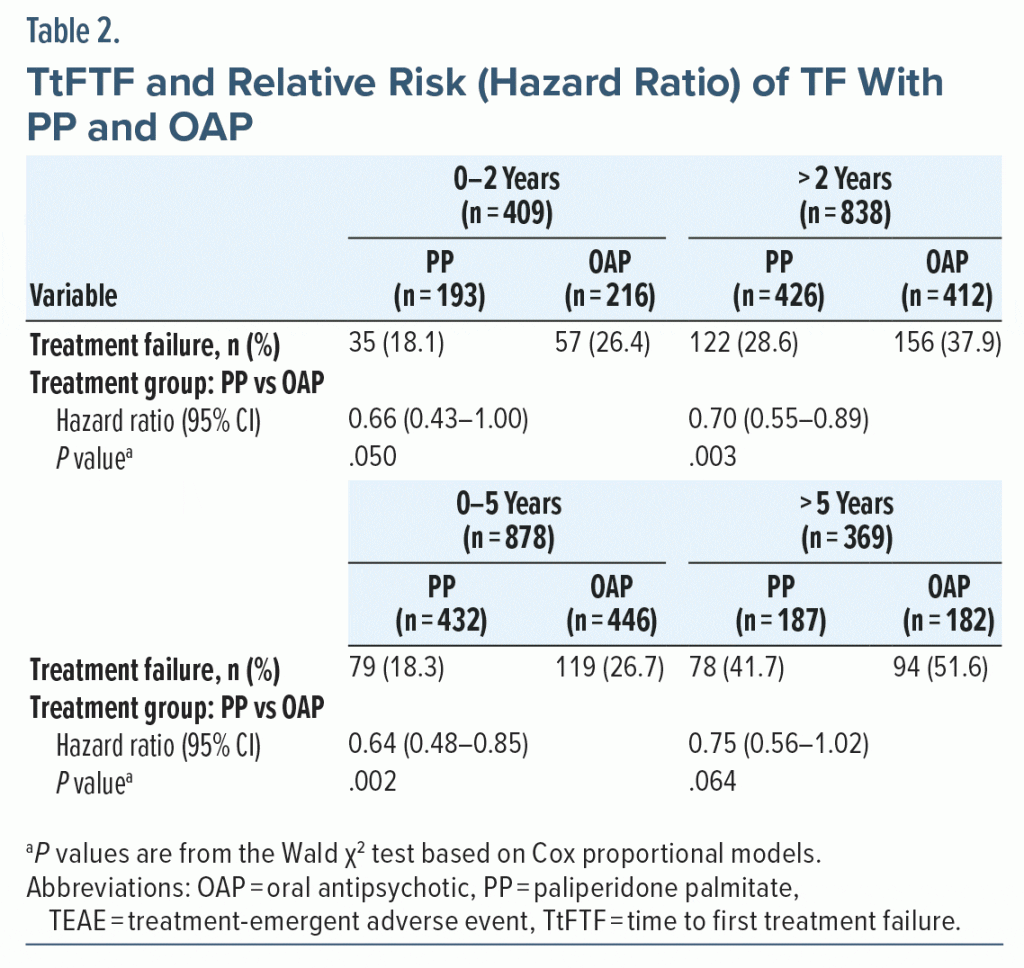
Treatment failure. There were fewer TFs observed with PP versus OAP in both the 0–3 years group (17.7% and 25.3%, respectively) and the > 3 years group (32.3% and 42.4%, respectively). TtFTF was significantly longer with PP versus OAP in both duration of illness groups (0–3 years group, HR = 0.67; 95% CI, 0.47–0.95; P = .026; Figure 2A; > 3 years group, HR = 0.69; 95% CI, 0.53–0.89; P = .004; Figure 2B); risk of TF with PP versus OAP was reduced by 33% in the 0–3 years group and by 31% in the > 3 years group. Similar results were observed when examining risk of TF at 2 years or 5 years (Table 2).
PSP scale. Overall, social functioning as determined by PSP scale scores was similar between PP and OAP in both duration of illness groups. From baseline to endpoint (15 months), mean PSP scale scores increased from 55.4 to 66.1 in the 0–3 years group and from 56.5 to 65.4 in the > 3 years group, respectively.
MSQ. Medication satisfaction as measured by the MSQ was generally similar across groups and increased slightly from baseline to endpoint (12 months). In the 0–3 years group, the mean (SD) MSQ score increased from 5.0 (1.10) to 5.2 (1.25) in the PP group and from 4.9 (1.02) to 5.1 (1.21) in the OAP group. A similar trend was observed in the > 3 years group, with mean (SD) MSQ scores increasing from 4.6 (1.36) to 5.1 (1.55) and 4.7 (1.28) to 5.2 (1.43) for the PP and OAP groups, respectively.
Safety Outcomes
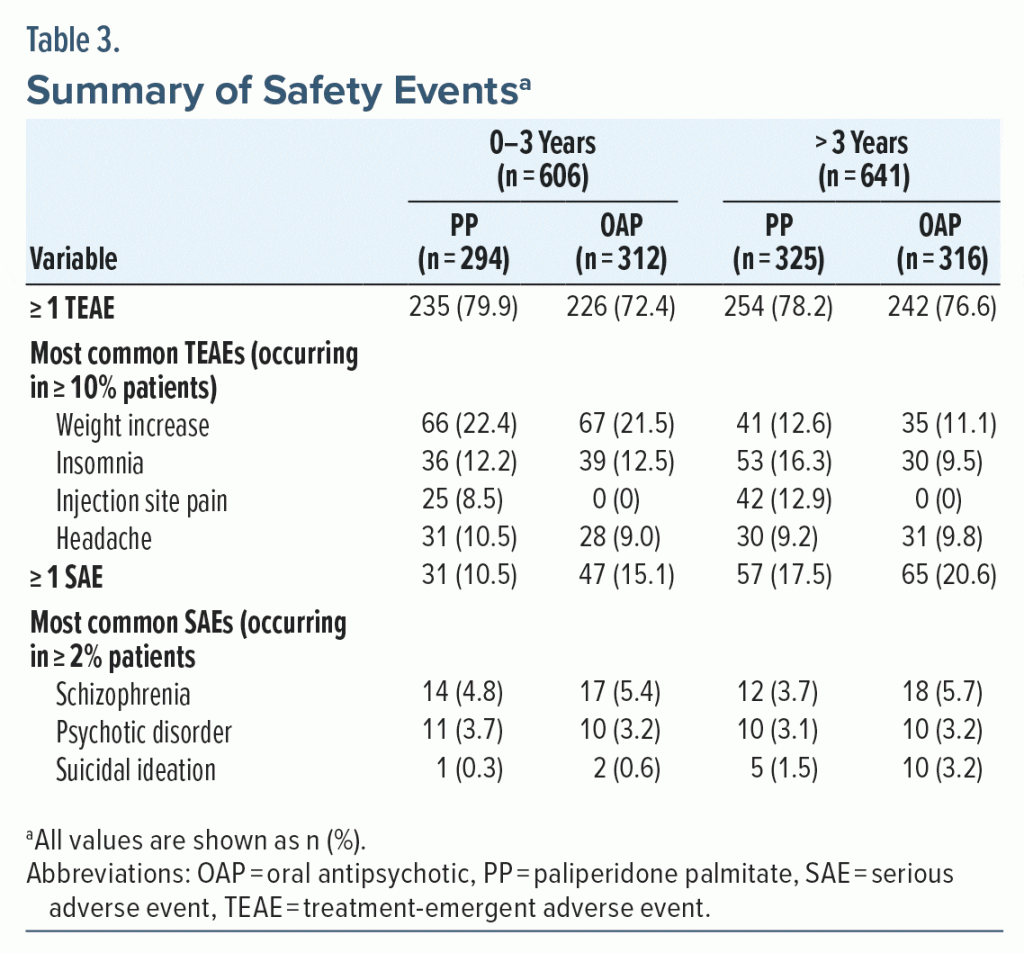
A total of 957 patients (76.7%) experienced a TEAE (0–3 years: PP, n = 235 [79.9%], OAP, n = 226 [72.4%]; > 3 years: PP, n = 254 [78.2%], OAP, n = 242 [76.6%]). The most common TEAEs (occurring in ≥ 0% of patients) were weight increase, insomnia, headache, and injection site pain (PP only) (Table 3). In the 0–3 years group, SAEs occurred in 31 patients (10.5%) and 47 patients (15.1%) in the PP and OAP groups, respectively; in the > 3 years group, 57 patients (17.5%) and 65 patients (20.6%) in the PP and OAP groups experienced an SAE, respectively. The most common SAEs were schizophrenia, psychotic disorder, and suicidal ideation (Table 3).
DISCUSSION
This patient-level post hoc analysis suggests that PP provides significant benefit in reducing TF or relapse compared with OAPs regardless of duration of illness. TtFTF was significantly longer with PP versus OAP treatment regardless of treatment duration. Consistent with the notion that early and sustained antipsychotic use is important for the treatment of people diagnosed with schizophrenia, greater benefit was observed when PP was introduced in patients with 0–3 years of illness compared with patients with > 3 years of illness. Additionally, social functioning (assessed using the PSP scale) was similar between the PP and OAP groups regardless of duration of illness. Furthermore, patients in all groups indicated they were generally satisfied with their study medication. TEAE rates were similar between the PP and OAP groups, and the most common TEAEs were weight increase, insomnia, headache, and injection site pain (PP only).
Previous studies have demonstrated that use of LAIs can result in significantly higher medication adherence rates and lower relapse rates compared with OAPs.11 Kane et al19 found that use of LAIs in patients with early-phase schizophrenia (defined as < 5 years of lifetime antipsychotic use) led to a significant and clinically meaningful 44% reduction in the incidence rate of first hospitalization. According to the “critical period” concept, there is a rapid progression of illness within the first 3–5 years following the initial presentation of disease, after which point disease progression plateaus.29 Therefore, therapeutic intervention during this critical period can often impact and even predict outcomes later in the course of disease.31,32 Prior studies have used cutoffs of 2, 3, or 5 years to differentiate early versus late intervention for schizophrenia. A conservative timeframe of ≤ 3 years was chosen for this study, which can be considered more stringent than studies that used a cutoff of 5 years. This cutoff also aligns with data from a previous study of LAI antipsychotics by Macfadden et al33 in which patients treated with risperidone long-acting microspheres who had a longer disease duration (> 3 years) were twice as likely to relapse compared with patients who were recently diagnosed with schizophrenia (≤ 3 years). Macfadden et al also demonstrated that recently diagnosed patients had better outcomes compared with patients who had a longer disease duration.33 These results provide further support that early stages of schizophrenia are a critical period for effective therapeutic interventions.
There are several limitations of this analysis. Patients were enrolled in clinical trials with specific inclusion and exclusion criteria; therefore, they may not be fully representative of the demographics of the real-world patient population with recent-onset schizophrenia. Observation time was limited by the designs of each study; a longer observation length may have revealed more substantial differences between duration of illness groups. Patients in DREaM were stabilized on OAPs prior to randomization, resulting in a large study effect and an initial improvement prior to receiving randomized treatment, which may have impacted the treatment effects observed in that study. The treatment discontinuation rate was higher in the > 3 years group compared with the 0–3 years group (46% vs 27.1%) and could have impacted the interpretation of results. Only a limited number of oral medications were compared; therefore, results may have differed if comparisons were made against other OAPs. Additionally, there are potential biases inherent in any study sponsored by a for-profit entity; however, these biases have been mitigated by rigorous oversight of the study and a thorough presentation of all methodology involved. Furthermore, there may be additional biases present in any clinical trial, such as the unintentional exclusion of individuals who may be hesitant to participate in clinical research and/or whose primary language is not English.
Schizophrenia is a complex and chronic disorder that requires both prompt and thoughtful consideration regarding treatment options.13 The results of this study highlight the potential benefit of implementing PP earlier in the course of schizophrenia in adults, although improvement in TtFTF was seen regardless of the duration of illness. Overall, PP was well tolerated, and no new safety signals were identified. Further research evaluating the use of PP among patients with varying durations of illness is warranted.
Article Information
Published Online: September 25, 2023. https://doi.org/10.4088/JCP.23m14788
© 2023 Physicians Postgraduate Press, Inc.
Submitted: January 6, 2023; accepted July 10, 2023.
To Cite: Lopena OJ, Alphs LD, Sajatovic M, et al. Earlier use of long-acting injectable paliperidone palmitate versus oral antipsychotics in patients with schizophrenia: an integrated patient-level post hoc analysis. J Clin Psychiatry. 2023;84(6):23m14788.
Author Affiliations: Janssen Scientific Affairs, LLC, Titusville, New Jersey (Lopena, Alphs, Johnston, Sliwa, Najarian, Starr); Neurological and Behavioral Outcomes Center, University Hospitals of Cleveland Medical Center, Case Western Reserve University School of Medicine and University Hospitals of Cleveland, Cleveland, Ohio (Sajatovic); Janssen Research & Development, LLC, Titusville, New Jersey (Turkoz); Cytel, Cambridge, Massachusetts (Sun). Drs Alphs and Starr were employees of Janssen Scientific Affairs, LLC, at the time studies were conducted.
Corresponding Author: Oliver J. Lopena, PharmD, Janssen Scientific Affairs, LLC, 1125 Trenton-Harbourton Road, Titusville, NJ 08560 (olopena@its.jnj.com).
Author Contributions: All authors provided direction and comments on the manuscript, reviewed and approved the final version prior to submission, made the final decision about where to publish these data, and approved submission to this journal. All authors had full access to the study data and take responsibility for data integrity and the accuracy of the analyses.
Relevant Financial Relationships: Drs Lopena, Johnston, Sliwa, and Najarian are employees of Janssen Scientific Affairs, LLC, and hold stock in Johnson & Johnson, Inc. Drs Alphs and Starr were employees of Janssen Scientific Affairs, LLC, at the time the study was conducted and hold stock in Johnson & Johnson. Dr Turkoz is an employee of Janssen Research & Development, LLC, and holds stock in Johnson & Johnson, Inc. Ms Sun is an employee of Cytel. Dr Sajatovic has received research grants from Centers for Disease Control and Prevention (CDC), International Society for Bipolar Disorders (ISBD), National Institutes of Health (NIH), Otsuka, and Patient-Centered Outcomes Research Institute (PCORI); has served as a consultant for Alkermes, Clinical Education Alliance, Health Analytics, Janssen, Lundbeck, Otsuka, Sunovion, and Teva; has received royalties from Johns Hopkins University Press, Oxford Press, Springer Press, and UpToDate; and has received compensation for preparation of/participating in continuing medical education activities for the American Academy of Child and Adolescent Psychiatry, American Epilepsy Society, American Physician Institute (CMEtoGo), American Society of Clinical Psychopharmacology, Neurocrine, and Psychopharmacology Institute.
Funding/Support: This study was funded by Janssen Scientific Affairs, LLC, Titusville, NJ.
Role of the Funders/Sponsors: The study sponsor was involved in the design and conduct of the study and the collection, management, analysis, and interpretation of data.
Previous Presentation: This work was previously presented as a poster at Psych Congress Elevate; June 3–5, 2022; Las Vegas, Nevada.
Acknowledgments: The authors thank Lindsey Kirkland, PhD, and Kevin O’Regan, PhD (ApotheCom, Yardley, PA), for editorial and writing assistance, which was funded by Janssen Scientific Affairs, LLC.
Additional Information: The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.
Clinical Points
- Early intervention in schizophrenia is critical; however, long-acting injectable antipsychotics (LAIs) are typically reserved for patients with a long history of schizophrenia.
- An analysis of 3 studies demonstrated that there was a benefit in initiating the LAI paliperidone palmitate earlier in the course of schizophrenia in adults.
Quick Links: